
The self-sustained fission of nuclei is commonly referred to as a chain reaction, as shown in Figure 22.31.įigure 22.31 A chain reaction can produce self-sustained fission if each fission produces enough neutrons to induce at least one more fission. Both factors affect critical mass, which is smallest for 239Pu. In particular, 235U and 239Pu are easier to fission than the much more abundant 238U. Additionally, some nuclides are easier to make fission than others. Some nuclides, such as 239Pu, produce more neutrons per fission than others, such as 235U. The minimum amount necessary for self-sustained fission of a given nuclide is called its critical mass. We can enhance the number of fissions produced by neutrons by having a large amount of fissionable material as well as a neutron reflector. Some neutrons escape the fissionable material, while others interact with a nucleus without making it split. However, not every neutron produced by fission induces further fission. Those neutrons have the potential to cause further fission in other nuclei, especially if they are directed back toward the other nuclei by a dense shield or neutron reflector (see part (d) of Figure 22.30). When a nucleus is split, it is not only energy that is released, but a small number of neutrons as well. Since fewer nucleons are in contact, the repulsive Coulomb force is able to break the nucleus into two parts with some neutrons also flying away.Īs you can imagine, the consequences of the nuclei splitting are substantial. Acting like a struck liquid drop, the nucleus deforms and begins to narrow in the middle. First, energy is put into a large nucleus when it absorbs a neutron. The imbalance of forces can result in the two ends of the drop flying apart, with some of the nuclear binding energy released to the surroundings.įigure 22.30 Neutron-induced fission is shown. As the nucleus elongates, nucleons are no longer so tightly packed, and the repulsive electromagnetic force can overcome the short-range strong nuclear force. This is why the model is known as the liquid drop model.
Difference between fission and fusion free#
The catalyst typically occurs in the form of a free neutron, projected directly at the nucleus of a high-mass atom.Īs shown in Figure 22.30, a neutron strike can cause the nucleus to elongate, much like a drop of liquid water. As a result, a physical catalyst is necessary to produce useful energy through nuclear fission.

And although it is true that huge amounts of energy can be released, considerable effort is needed to do so in practice.Īn unstable atom will naturally decay, but it may take millions of years to do so. Given that it requires great energy separate two nucleons, it may come as a surprise to learn that splitting a nucleus can release vast potential energy.
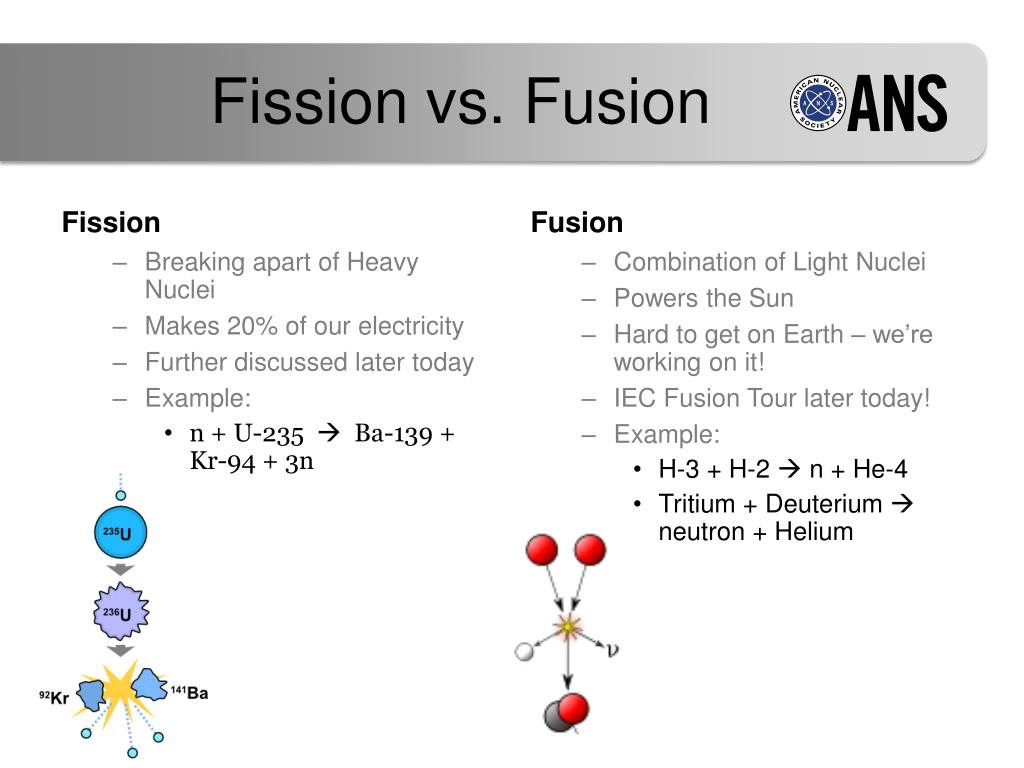
In simplest terms, nuclear fission is the splitting of an atomic bond.


 0 kommentar(er)
0 kommentar(er)
